Preventing Childhood Hearing Loss Worldwide
Michaela Martinez
Local and global collaborations help BME Design Fellows make a low-cost, affordable, and easy-to-use screening tool for hearing loss in children
Jacklyn “Jackie” Herzberg was in her junior year as a biomedical engineering (BME) student at Duke University when she approached her professor, Mark Palmeri, with a question. Herzberg had just finished the first sequence of BME Design Fellows, a three-semester program that enables students to design and test medical instruments based on needs identified by Duke Health clinicians.
Herzberg was looking for an independent study project that would help her gain additional experience in the world of medical device design. But she didn’t know that Palmeri, a professor of the practice in biomedical engineering, had just the project in mind––one that would span the Atlantic ocean and could help prevent hearing loss worldwide.
Ears Under Pressure
It’s a fact of life that most children suffer through an ear infection, with the National Institutes of Health (NIH) estimating that 5 of 6 children in the U.S. alone experience at least one ear infection before their third birthday. Usually, the signs of infection are clear––children have a hard time hearing from the infected ear, have trouble sleeping, spike a fever and experience pain that triggers at least a few bouts of tears.
But many children in under-served and rural communities don’t ever receive proper treatment.
“When that happens, the infection and fluid can sit in the ear for a while and cause scarring to the eardrum,” explained Palmeri. “And that scarring can lead to hearing loss that can persist into adulthood.”
One in five people around the world have some form of hearing loss, and according to the World Health Organization, nearly 60 percent of hearing loss in children is preventable with proper screening.
The current screening method for ear infections involves a tool called a tympanometer, which tests how well the middle ear works by measuring how the eardrum moves. During the exam, an audiologist inserts a probe into the ear that creates a seal and controls the pressure in the ear canal. A speaker on the tool then plays a tone at specific pressure levels, and a microphone records how the eardrum moves in response.
“In a healthy ear, the sound from the speaker should hit the eardrum and reflect back towards the microphone,” said Palmeri. “When pressure is neutral, the ear drum should move to allow sound through. But if you have pus or some other material behind your eardrum, the sound gets reflected back. That’s when the child needs to see a doctor.”
While there’s no doubt tympanometers are effective, their reach is limited. The devices cost thousands of dollars, and a trained audiologist is required to read and assess the measurements. These factors make them more likely to be available in cities with robust healthcare systems.
This is an issue that Samantha Robler, a clinical and research audiologist, and Dr. Susan Emmett, then an associate professor of surgery and global health at Duke, are well aware of. The duo had been working on a large, randomized trial to investigate childhood hearing loss in rural Alaska when they realized that a mobile, easy-to-use version of a tympanometer was necessary to help prevent hearing loss in underserved populations.
“Hearing loss is extremely common in rural areas. The World Health Organization estimates that 80 percent of hearing loss worldwide is in under-served communities––the exact places that have the least access to care,” said Dr. Emmett, now the founder and director of the Center for Hearing Health Equity (CCHE) at the University of Arkansas for Medical Sciences. “This is particularly concerning for children, because we know that approximately 75 percent of hearing loss in children in underserved communities is due to ear infections and is preventable.”
But Emmett and Robler also knew they needed engineers if they wanted to make this idea a reality. In 2020, Palmeri, Emmett, Robler formed an interdisciplinary team of physicians, engineers and students and received a grant to jump-start the project, officially called the mHealth Tympanometer.

Back to Basics
The mHealth Tympanometer needed to be more affordable, and it needed to be so easy to use that a professional audiologist wasn’t necessary. These needs became the two arms of the project.
“When we started, we asked, ‘What bells and whistles can we throw out to reduce it to its barest bones?’” said Palmeri. “We wanted our design to be as off-the-shelf friendly as possible. Some tympanometers can cost up to $5,000. We want ours to be in the range of $50.”
First, the team wanted to create a machine learning algorithm that could effectively interpret data and limit the need for an audiologist. Palmeri recruited Felix Jin and Ouwen Huang, two MD-PhD students who were working with Palmeri at the time, to develop the algorithm.
With support from the Duke Global Health Institute, Jin and Huang developed a deep learning model using tympanometry data collected by both trained audiologists and laypeople from school-aged children in rural Alaska. By comparing these readings, the team taught their algorithm how to interpret the range of data with the same sensitivity as a trained audiologist.
“In our study population of rural Alaskan children, ten percent of examined ears had an abnormal tympanometry result that indicated some form of damage, and tympanometry improved the sensitivity of school hearing screening by 20 percent,” said Jin, now a diagnostic radiology resident at the Duke University Medical Center. “These results helped improve our model training and performance, but it also highlights the critical need and urgency for better treatment. This clear clinical need helped motivate us and focus our efforts.”
“Ouwen and Felix’s work was significant because it showed us that an automated machine learning tool could make it possible for someone like a school nurse or a teacher to effectively perform screening tympanometry,” said Palmeri. “And because we could leverage the processing power of a regular Android phone, it was also cost-efficient.”
Ideally, the device would collect the data and send it to a phone via Bluetooth, where the machine learning algorithm would analyze the reading and display the results.
But the design of the device itself was less straightforward.
“Early days were a hot mess because it was during COVID,” Palmeri said. “Supply issues were common, and Susan and Samantha left Duke to start the Center for Hearing Health Equity at the University of Arkansas for Medical Sciences, so they were working with us remotely.”
But there were silver linings despite the chaos. Groups of BME Design Fellows cycled through the project, helping to design an effective exterior for the tympanometer that served as the springboard for future iterations. The team also received both R21 and R33 grants from the NIH, which provided additional funding to support the prototyping process.

“I really got thrown into the project,” said Herzberg, who joined the team in 2023 at Palmeri’s recommendation. The BME Design Fellow realized that she’d need to learn advanced engineering skills on the fly if she wanted to make helpful contributions. “Initially, I was adapting things that students had worked on at earlier points in the design process, but once I got more comfortable, I started developing more of the firmware and took over testing the electronics.”
By the end of her junior year, Herzberg was the de facto leader of the project, and she was even able to continue her work during her summer internship at Blur Product Development in nearby Research Triangle Park. This was especially helpful, as many aspects of the device needed to be custom made––something that wasn’t always achievable in an academic setting, even with the resources available at Duke.

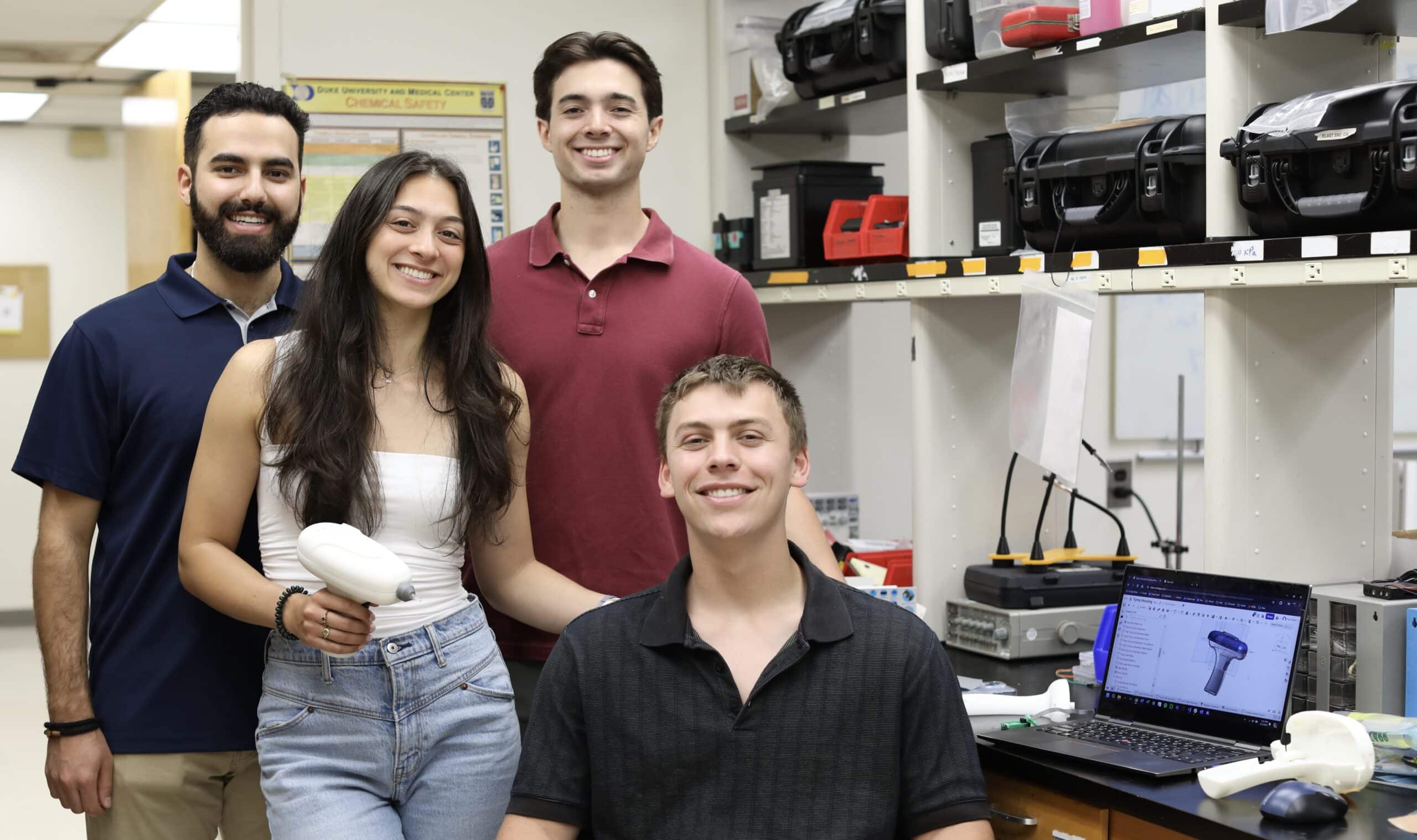
Unlike other portable tympanometers, which are typically tabletop or awkward portable devices with a screen and a probe on a cable that fits in the ear, the mHealth tympanometer has a sleek design that fits into the palm of the hand the way a glue gun does. An exterior case of 3D-printed white plastic holds the microphone, speaker, pressure sensor, and an actuator and syringe, which are all connected by wires and tubing. The top of the syringe extends at the front of the device, and a silicone ear tip can be added to its opening to create a seal when inserted.
The device was fully functional by the fall semester of 2023––though, Palmeri says, the team knew there were still some kinks to work out. But by then they had new collaborators ready and willing to help.
Forging Global and Local Connections
“I was really inspired by Jackie’s enthusiasm for this project,” said Dmitri Morales, a BME senior and Design Fellow, who’d been recruited by Palmeri to work on the tympanometer. “I knew I had a love for engineering, but working on this project really inspired a passion for medical technologies.”
Morales wasn’t the only BME Design Fellow to join the team that semester––Kyle Duerr and Tasman Miley, also BME seniors, had jumped at the opportunity to join the project after hearing about the device from Herzberg and Palmeri.
“We were all friends with Jackie, and it seemed like she’d been having a lot of fun working on the tympanometer,” said Duerr. “I was really excited to get to work on a project that was clearly going to make an impact.”
Although Herzberg oversaw every aspect of the project, each new teammate focused on improving a different component of the functional device. Deurr helped streamline the code and reformatted the enclosure to fit new electronic components. Miley was tasked with adjusting the syringe and tip of the device to ensure that it could be easily removed for cleaning. Morales adapted the Bluetooth in the tympanometer so it could send more information to an app the team developed in partnership with hearX, a South African company that creates mobile apps and tools for hearing screening and diagnostics.
“I think we realized how big the team had gotten when we had to arrange meetings across four time zones,” said Herzberg. “We had Dr. Emmett and Dr. Robler in Arkansas, our team in Durham, an audiologist in Hawaii, and the team at hearX in South Africa.”

The prototype was finally put to the test beginning March of 2024, when Herzberg flew to Pretoria, South Africa to test and troubleshoot the device with the hearX team. Robler, an assistant professor at the University of Arkansas for Medical Sciences and associate director of the CCHE, is leading the first-in-human study with the device in Arkansas. This effort will soon be followed by a larger community-based study with 500 preschool children in South Africa.
Moving forward, the team will use these data to optimize and make necessary changes as they finalize the device. They’ve received a grant from the Duke-Coulter Translational Partnership to help support these efforts.
“Once we’ve locked in the design, we’ll apply for FDA clearance so we can hopefully get the tool approved domestically. We also made sure that all intellectual property was open-sourced and costs zero dollars to license from Duke. There’s a global need for a tool like this––our data and design are things we want people to use,” said Dr. Palmeri.
Although the student team all graduated from Duke in 2024, Herzberg will continue to work on the device at Blur––this time on staff as a research and development engineer. Blur will continue to serve as an engineering consultant and help the team craft the final device and determine how to scale up their design if it’s approved.
“This entire project was so important for my academic and professional development,” she said. “I think it’s unusual for students to lead a big design project like this, but I’m so grateful to my friends, our collaborators, and Dr. Palmeri for being a great influence and helping me seize this opportunity.”

